 |
The
WindFuels™ Primer
- Basic Explanation
for the Non-scientist
The
WindFuels™ Basic Process Explanations.
WindFuels™. Now that is a word you have not heard before.
So what are WindFuels™? The concept is really not complicated.
We will use energy generated by wind to power processes that will
recycle waste
carbon dioxide into transportation fuels for automobiles, like
diesel, ethanol or gasoline. We can also make fuels like jet fuel
and
propane.
(We are talking mostly about fuels, but the FTS process can also
produce
ethylene and propylene which are used to
make plastics – used in everything from textiles to
tables.)
We'll recycle CO2 from
power plants or other exhausts (which release CO2into
the air, contributing
to global warming). Because we have removed the CO2 from
the air to make the fuels, using (burning) WindFuels releases no
new carbon, making it a carbon
neutral process. Replacing oil with WindFuels will reduce total
CO2 emissions
by 40%.
No experienced chemist has doubted that it is possible to convert
CO2 to fuels. The problem has
been that prior proposals for doing this conversion have had
efficiencies of only 20% to 30%.
The combination of the eight
major technical advances we have made over the past five years
will now permit this conversion
to be done at 60% efficiency. That’s high enough for carbon-neutral
fuels made from waste CO2 to easily
compete with petroleum on a cost basis, especially when the input
energy is from excess wind energy in the middle of the night.
What we are doing is not magic. It is just good chemistry, physics,
and engineering. Because we are using the carbon from waste CO2 rather
than coal, we have to add a lot of energy from wind. However, when
all the processes are properly optimized, the cost of this energy
becomes affordable. It is a small price to pay to dramatically
reduce greenhouse gases in the atmosphere and provide a limitless
supply of clean transportation fuels.
Fuels like ethanol, gasoline and jet fuel are hydrocarbon fuels.
Hydrocarbons and alcohols are chemicals that contain hydrogen (H),
carbon (C) and oxygen (O). We will use water (H2O)
and the waste (polluting) carbon dioxide (CO2)
from power-plant smokestacks to provide the carbon, oxygen, and
hydrogen needed to make fuels like ethanol (C2H5OH)
and gasoline (C8H18).
Here's How: (Later
on this page, we’ll explain each of these processes -
and we will try to explain them in a clear way.)
Wind
Farms

|
1. Wind Farms generate
electricity for electrolysis and other processes. |
|
2. Electrolysis is the process in which electric current
is passed through water (H2O) to break the bonds
between the hydrogen and the oxygen, yielding hydrogen (H2) and Oxygen
(O2). |
RWGS & FTS Plant
 |
3. Reverse Water
Gas Shift (RWGS) is used to
produce carbon monoxide (CO) from carbon dioxide (CO2). |
| 4. In a widely used process
called Fischer
Tropsch Synthesis (FTS),
the liquid fuel production from hydrogen (H) and carbon
monoxide (CO) occurs. |
WindFuels™

|
5. The resulting products will
fuel our cars, trucks or
jet planes and are:
Carbon neutral
(WindFuels do not release new CO2 into
the air. The carbon was recycled from exhausts.)
Renewable (Both wind and CO2 are
replaced. In contrast – oil and coal which are
used up are not renewable.)
Economical (competes on a price basis)
Contributing to Energy Independence!
|
Overview of FTS (Fischer
Tropsch Synthesis)
The liquid fuel production from hydrogen, carbon, and
oxygen occurs in the Fischer Tropsch (FT) reactor. FTS has been
used in commercial
production
of liquid
fuels of
all types from coal or natural gas (gas) for over 60 years.
FTS was used in Germany during WWII to generate
fuels when crude oil was scarce, so the process is not
new.
The process has not been used much in
the United States because oil was cheap and plentiful. Utilization
of the FTS processes has been increasing, as coal and
gas are now much cheaper than oil. (If we use waste CO2 in
place of gas or coal in FTS, we can supply fuel and reduce
the CO2
in the air.)
The FTS process uses catalysts to efficiently convert a feed
mixture of carbon monoxide (CO) and hydrogen (H2)
to hydrocarbons of all
types. Different catalysts and different operating conditions
can help “select” for higher yields of some hydrocarbons
than others, but there will always be a mixture of different
products created by the reaction:
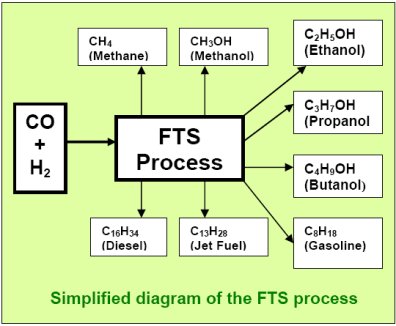
In conventional FTS, the syngas is obtained from high-temperature
reforming of coal or methane. Usually, the FT catalysts and conditions
(pressure, temperature, and mixture) have been chosen to obtain mostly
gasoline, diesel, and waxes from the FT reactor. Recent progress
in the catalysts and conditions now allow high yields of ethanol,
propanol, and butanol also.
Getting clean hydrogen - Electrolysis
Electrolysis is used rather than fossil fuels to generate the hydrogen
for our WindFuels. Electrolysis is the process in which electric current
is passed through water (H2O) to break the
bonds between the hydrogen and the oxygen, yielding hydrogen (H2)
and Oxygen (O2).
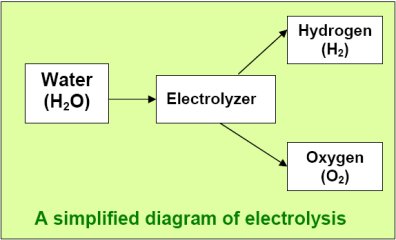
Electrolyzers
for efficiently splitting water into high purity hydrogen and
oxygen have been in industrial production for decades. A solution
of potassium hydroxide (KOH) in water is used because it has
low
resistivity and thus lower power loss. The addition of electrons
at the negative electrode (also called the cathode) produces
hydrogen gas (H2) and hydroxyl
ions (OH-), which remain in the solution. At the positive electrode
(also
called the anode), electrons are removed
from OH- ions, producing water (H2O)
and oxygen (O2). A membrane that
is permeable to the OH- ions (and possibly to water too)
separates the two electrodes to keep the gases from mixing
while allowing the
electrical current to flow through it on the charge carriers.
In practice, the solutions on both sides are continually flowing
to
maintain the desired salt concentrations. The two gases produced
also contain a lot of water vapor (which is easily separated)
but only minute traces (easily under 0.1%, and sometimes
under 0.01%) of
other impurities (primarily the other major gas, either O2
or H2).
Efficiency of commercially available 2 MW (megawatt) electrolyzers
has typically been 73%. Laboratory experiments have exceeded
85%
at higher
pressures and lower current densities, and we have shown that
the waste heat
(at 160 0C) can be utilized at
30% efficiency. Total system efficiency of a 250 MW electrolysis
system may eventually approach
90%.
A quick note about water, the FTS process will require about
5 gallons of water for every 3-4 gallons of produced fuels, which
is at least
an order of magnitude less than the water requirements for
biofuels. Using reverse-osmosis, water sources as impure as seawater
can be
used, which would add only $0.01/gallon to the cost of the
fuel produced. Water will not be a limitation.
Getting
the CO: The Reverse Water Gas Shift (RWGS)
The next step is to efficiently get the carbon monoxide
(CO) needed in the syngas from CO2.
There is a very robust and efficient reaction that has been known
for the past century as the water gas shift reaction (WGS). This
is used in fossil-fuels FTS to generate hydrogen by combining
CO (from fossil fuels) with steam at high temperatures (400-800
0C)
to form hydrogen and carbon dioxide.

The fossil fuels FTS
systems have no trouble getting carbon monoxide, and use excess
carbon monoxide to get the hydrogen they need for proper syngas
mixtures. The reverse is true for clean, renewable WindFuels. Through electrolysis,
we can efficiently
get all the H2 we need, but we
need an efficient way of getting the CO.
The reverse of WGS reaction, known as the reverse water gas
shift (RWGS) provides a robust method of producing CO
and water from CO2 and H2.
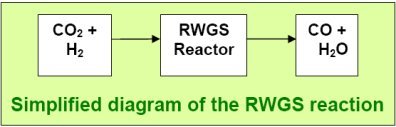
Getting this reaction to achieve high yield of CO at high efficiency
with low production of unwanted methane (CH4)
has previously been a challenge, but we have shown elsewhere
in detail
how this can now
be achieved achieved at very high efficiency.
The syngas (remember, the CO plus H2 mixture)
then goes to the FT reactor, where it is adsorbed onto the surface
of the catalysts
(often
small metal particles), where it is reformed into hydrocarbons
(such as gasoline, propane, and diesel), alcohols (including
ethanol and
propanol), water, and waste heat. As these reactions are
exothermic (heat is released), they proceed readily. The reaction
efficiencies
here are in the range of 70-85%, depending on the compound
that is formed, with the higher efficiencies being for the light
alcohols
(methanol and ethanol). We have shown elsewhere in detail
how the waste
heat from the reactor can be utilized at over 40% efficiency.
The output from the FT reactor includes the desired products
(alcohols, jet fuel, propane, etc.) along with a lot of
unreacted inputs
(CO and H2) and some undesired
products – water, CO2,
and methane. One of the most important keys to achieving
high system
efficiency
is devising extremely efficient methods of separating and
recycling the unwanted components. We have developed important
improvements in separations and recycling. Elsewhere
we have shown how this can be done.
Fossil-Based
FTS is dirty. Windfuels are a path to true Global Warming
Mitigation.
The biggest problem with fossil-based FTS is that an enormous amount of polluting
CO2 is released – especially if coal is
used. For every kg of coal used for coal-to-liquids (CTL) diesel, 2.2 kg
of CO2 are emitted and 0.3 kg of fuel is produced.
(Even NG-based FTS results in about 25% more CO2 total
release than simply using conventional oil.) WindFuels uses similar FTS processes,
but begins with carbon-neutral “syngas” (the feed mixture of
CO and H2) made from water (H2O)
and waste CO2 (from coal plants). This can be
done at very high efficiency with zero net carbon release, as we show in
detail elsewhere on this website and summarize below.
| Below: Since
the CO2 was removed from the air (or smokestacks)
to make the WindFuels, no new CO2 has been
released. The carbon was recycled. The net carbon from
WindFuels is zero. |
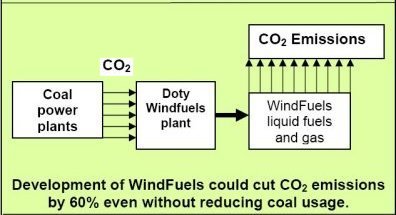
Some may say that the CO2 from
coal is eventually released, and this is therefore not carbon
neutral. However, many of those same people have probably either
bought carbon offsets or at least looked into the idea. The
principle of carbon offsets is to reduce carbon emissions elsewhere
to offset the carbon you are generating. Well, the coal from
the coal power plants (which provides electricity to hundreds
of millions of homes) emits billions of tons of CO2.
If we recycle that CO2 to produce
WindFuels, it will still eventually be emitted, but oil and
natural gas – as well as much more environmentally destructive
fuels such as coal-to-methanol, tar-sands fuels, and oil-shale
fuels – are NOT burned and are therefore NOT emitting
CO2… which reduces overall
greenhouse gas emissions.
Eventually, the CO2 can be taken from the atmosphere
rather than from smokestacks, but that will be more expensive. Today, using
CO2 from the atmosphere might make the WindFuels
40% more expensive. Thirty years from now, we’ll probably be able to
do it for just an 8% cost penalty. We can’t wait 30 years to get started.
We'll start with CO2 from smokestacks.
Perfectly Solving the Grid
Stability Challenge.
We’ve all probably heard it will not be possible to stabilize the power
grid if much more wind energy is added, and the result would be frequent regional
grid failures and blackouts. Without a solution to the energy storage problem,
that would be true. The electric grid stability challenge arises from changes
in grid supply (power plants, wind, and solar) not being able to follow the changes
in grid demand (from users) quickly enough. Wind power is often greater in the
middle of the night when demand is minimal. “Clean coal”, nuclear,
and many of the older natural gas power plants take many hours to turn down,
and there is not a cost effective method of storing energy other than pumped
hydro storage, which is not an option in most areas. (Compressed air energy storage,
CAES, will be either very expensive or quite inefficient.)
WindFuels will only draw
power during off-peak hours when there is excess renewable
energy available at very low cost. Off-peak power rates are often
under
15% of peak rates to encourage more use of it. The WindFuels electrolyzer can
respond within milliseconds to changes in supply and demand. It will completely
solve the grid stability problem by storing the excess peak grid energy temporarily
in compressed hydrogen and then converting it to liquid fuels (which are easily
stored and distributed) at a fairly steady rate around the clock. Storing enormous
amounts of energy in hydrogen is considerably more expensive than storing energy
in liquid fuels, but storing enough hydrogen to keep the FTS
plant
going steadily around
the clock (to efficiently convert the hydrogen to liquid fuels) will not be
either too expensive or too risky.
Improving
the competitiveness of fuels from CO2.
The Windfuels process seems unquestionable destined to
be the dominant, sustainable solution for transportation fuels
in the future, but electrolyzers today are
still expensive. Hence, the capital outlay for the electrolyzers (for perhaps
the next five years) may be beyond what most investors wish to consider in today’s
risk-averse world.
That has motivated us to begin developing a less expensive “bridging” approach
to synthesizing fuels from a combination of CO2,
methane (from shale gas), water, and renewable energy. The outcome of this research
is a process we have dubbed
CARMA-GTL, for Carbon dioxide Advanced Reforming of Methane Adiabatically, with
GTL. As explained in a paper recently presented at the national ACS meeting,
as long as low-cost natural gas is available, our CARMA-GTL process reduces the
electrolyzer requirements by a factor of three to ten while actually increasing
plant efficiency. Since most of the carbon in these fuels comes from shale gas
(only a minor fraction comes from CO2), these fuels
are only slightly carbon neutral (like most biofuels). However, the CARMA-GTL
plants will be much less
expensive than Windfuels plants, and they will be able to steadily transition
to using more renewable energy and less shale gas. Developing this technology
will begin to drive the cost of electrolyzers down and allow investors to become
more comfortable with coming Windfuels paradigm.
|
 |
 
 
Western
Research Institute's
Test Fischer Tropsch System


|
| Better
than sequestration?
Sequestering a ton of CO2 prevents
one ton of CO2 from being emitted into the
atmosphere, adding a significant cost burden.
Pumping one ton of CO2 into
a WindFuels plant would profitably create about 170 gallons
of liquid fuels (~0.56 tons), which keeps additional
fossil fuels from being consumed .
Since
the CO2 is removed from the air (or smokestacks)
to make the WindFuels, no new CO2 has
been released. The net carbon from WindFuels is essentially
zero.
Net CO2
added to the atmosphere
for 1 ton of various liquid fuels:
|
FUEL
|
CO2
|
| WindFuels |
0.2 tons
|
| Ethanol from established corn fields |
2.9 tons
|
| Conventional Oil |
4.4 tons
|
| Deep Sea Oil |
5.3 tons
|
| Heavy Oil |
5.7 tons
|
| Oil Shale, ICP |
6.0 tons
|
| Tar Sands |
6.0 tons
|
| Oil Shale, ATP |
6.5 tons
|
| Coal-to-liquids |
10 tons
|
Shockingly,
newly plowed grasslands converted to crops
releases tons of CO2.
(see Science 319, 1235-1238,
Mar 21, 2008.)
|
| |
| We
are using wind energy because it is the most cost effective
renewable power source in the United States. In some countries,
another renewable energy source like solar or geo-thermal
would be more appropriate. |
|
 |
|
  |
